When it comes to chemistry, understanding solution concentrations can feel like deciphering a foreign language. Whether you’re a student, researcher, or just someone with a curious mind, grasping the concept of dilutions is essential. The dilution formula is your trusty guide through this complex world. It simplifies the process and helps you achieve accurate results every time.
Have you ever found yourself staring at a lab experiment or recipe and wondered how to mix solutions correctly? You’re not alone! Many people struggle with these calculations, but fear not—this guide will break down everything you need to know about solution concentrations and how the dilution formula works. Get ready to dive into an easy-to-follow step-by-step approach that demystifies dilutions once and for all!
Understanding Solution Concentrations
Solution concentrations refer to the amount of solute dissolved in a solvent. This measurement is crucial for various applications, from laboratory experiments to cooking.
In chemistry, a solute is the substance being dissolved, while the solvent does the dissolving. Understanding this relationship helps clarify how solutions behave.
Concentrations can be represented in several ways—molarity, molality, and percentage concentration are just a few examples. Each unit serves different purposes depending on what you need.
Knowing concentration allows scientists and students alike to predict how substances will interact. It also guides proper dosage in pharmaceuticals or safe mixtures in industrial processes.
Grasping these concepts lays a strong foundation for mastering more complex topics related to solutions and reactions.
Types of Solution Concentration Units
Solution concentration can be expressed in various units, each serving a different purpose. Understanding these units is essential for accurate measurements and effective communication in scientific contexts.
Commonly used units include molarity (M), which measures the number of moles of solute per liter of solution. This unit is prevalent in laboratory settings due to its straightforward application in chemical reactions.
Another important unit is mass percent, indicating the mass of solute relative to the total mass of the solution. It’s useful when dealing with solutions where precise weight measurements are crucial.
Volume percent also comes into play, particularly in industries like food and beverage. It represents the volume of solute as a percentage of the total solution volume.
Parts per million (ppm) or parts per billion (ppb) are often employed for very dilute solutions, allowing scientists to communicate tiny concentrations effectively without confusion. Each unit has its specific applications tailored to distinct needs across various fields.
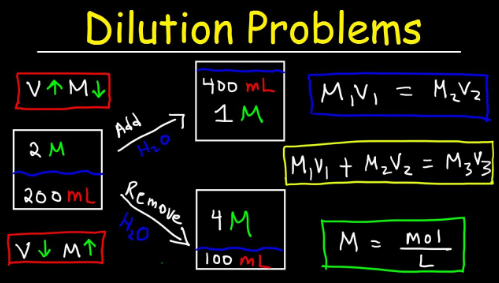
The Dilution Formula Explained
The dilution formula is a powerful tool in chemistry, helping to determine how much of a solution you need to create a desired concentration. It’s based on the principle that when you dilute a solution, its total amount remains constant.
The most common form of this formula is C1V1 = C2V2. Here, C1 represents the initial concentration and V1 is the initial volume. Conversely, C2 stands for the final concentration while V2 denotes the final volume after dilution.
Using this equation simplifies complex calculations and allows for precise adjustments in mixture concentrations. This makes it indispensable for labs where accuracy matters greatly.
Understanding how to manipulate these variables opens doors to many applications—be it preparing chemical reagents or mixing solutions in biology experiments. Knowing your starting point can lead you smoothly toward achieving your target dilution with confidence.
Step-by-Step Guide to Using the Dilution Formula
Using the dilution formula is straightforward once you grasp the basics. Start with identifying your initial concentration and volume. This will be your starting point.
Next, determine the desired final concentration. Knowing how concentrated you want your solution is crucial for accurate calculations.
Then, apply the dilution formula: C1V1 = C2V2. Here, C1 represents your original concentration, V1 is the volume you’re looking to find, C2 is your target concentration, and V2 stands for the total volume after dilution.
Rearranging this equation helps solve for V1 if needed. Just plug in what you know and perform simple algebra to isolate V1.
Measure out the calculated volume of solute and add it to enough solvent until reaching your final desired volume (V2). It’s that easy!
Examples of Dilutions in Real Life
Dilutions are all around us, often in ways we don’t immediately recognize. For instance, when you add a splash of lemon juice to water for a refreshing drink, you’re creating a diluted solution. The intensity of flavor decreases as the concentration lessens.
In the world of medicine, pharmacists prepare dilutions for liquid medications. This ensures patients receive safe and effective doses tailored to their needs.
In laboratories, scientists routinely dilute solutions for experiments. A concentrated acid might be mixed with water to achieve the desired pH level essential for reactions.
Even in cooking, recipes often call for diluted ingredients like broth or stock. Adjusting these concentrations can enhance flavors without overwhelming dishes.
These examples illustrate just how vital understanding dilutions is across various fields—from culinary arts to healthcare and scientific research.
Common Mistakes and How to Avoid Them
When working with the dilution formula, mistakes can easily happen. One common error is miscalculating volumes. It’s crucial to double-check your measurements before proceeding. Even a small discrepancy can lead to significant changes in concentration.
Another frequent issue is forgetting to use consistent units. Always ensure that you’re using the same measurement system for both volume and concentration. This will save you from unnecessary confusion and inaccuracies.
Also, don’t overlook temperature effects on solutions. Some substances behave differently at various temperatures, altering their effective concentrations.
Be cautious with labeling containers after dilution. Clear labels help prevent mix-ups later on when you’re handling multiple solutions simultaneously.
Staying organized and meticulous will enhance accuracy in your dilutions and experiments.
Tips for Accurate and Efficient Dilutions
To achieve accurate dilutions, always use precise measuring tools. Graduated cylinders and pipettes provide more accuracy than standard cups or spoons.
Ensure your workspace is clean and organized. A clutter-free area reduces the risk of contamination and mistakes during preparation.
When mixing solutions, add solute to solvent gradually. This prevents sudden reactions that can lead to errors in concentration.
Label all containers clearly with the solution’s name, concentration, and date prepared. This practice helps avoid confusion later on.
Always double-check calculations before preparing your dilution. A small error in volume can significantly affect results.
Take time to familiarize yourself with the properties of both solute and solvent used. Understanding their behaviors will enhance your overall efficiency when performing dilutions.
Conclusion
Understanding solution concentrations is crucial for various fields, from chemistry to pharmacology. Knowing how to work with different types of concentration units can significantly ease your lab work or any scientific endeavor.
The dilution formula serves as a key tool in mastering these concepts. By grasping its components and learning how to apply it, you empower yourself to create precise solutions tailored to your needs.
Real-life applications are abundant, whether you’re preparing a saline solution for medical use or mixing chemicals in an experiment. Being aware of common mistakes allows for more accurate results while following best practices ensures efficiency.
With the right knowledge and techniques at your disposal, you can tackle dilutions with confidence and clarity. Embracing this understanding opens up new avenues for experimentation and innovation in any project requiring precise liquid measurements.